 The Science Of Safety In Medical Physics
The Science Of Safety In Medical Physics
Highlights From Today’s Cutting Edge Medical Physics Research
Medical physicists need no convincing of the importance of quality and safety improvement – it’s in our DNA. The challenges lie in how to achieve quality improvements effectively, how to rigorously assess the effect changes in technology and process have on quality, and how to prove the value of improvements.
The growing appetite for safety research in medical physics, says Eric Ford, Ph.D., is evident in federal research grants awarded to medical physicists for research in quality and safety improvement in radiation therapy and diagnostic radiology.
“In recent years a number of quality-improvement tools have been developed, but the challenge is to effectively measure the impact these tools are having on patient care,” said Ford. “This is something that hasn’t been done in our field. We’ve traditionally been tool builders and know at an intuitive level that these tools should be beneficial, but we haven’t produced the data to back it up. But the field is evolving and recent work sets out to measure the impact of such tools through a variety of studies with various endpoints.”
Drawing a straight line from safety initiatives to improved care is key. Quality assurance research in radiation therapy, for example, has shown a clear link between quality metrics and improved outcomes for patients, notes Ford. This has been demonstrated in clinical trials from cooperative oncology groups.1 Such studies include review of hundreds of patient cases to provide statistical power demonstrating that patients with “high quality plans” have better outcomes, survive longer, experience better local control and benefit from lower toxicities.
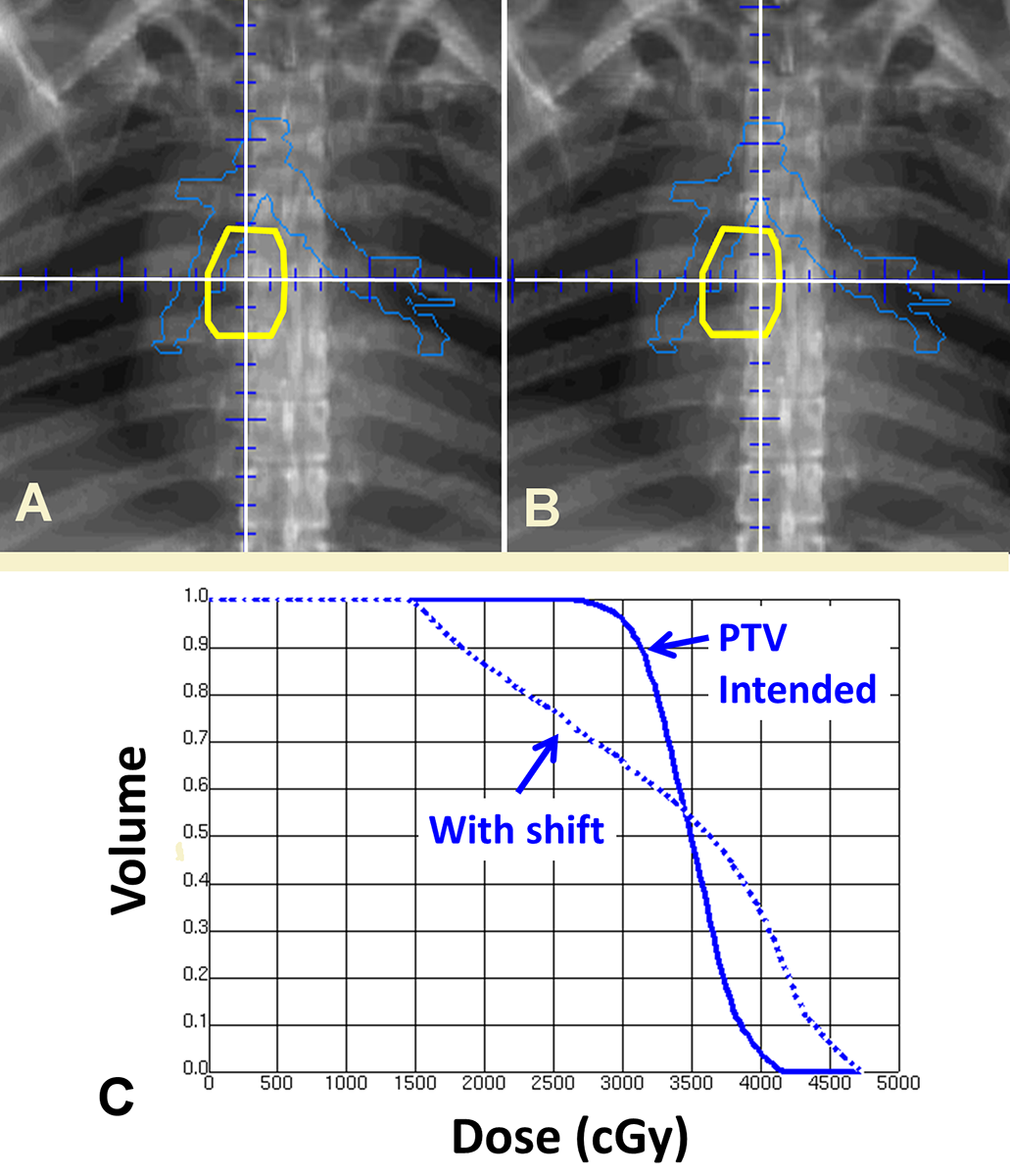
But how does one guarantee a “high quality” plan for each patient? In clinical trials this often is accomplished by outside audits of the plan (sometimes in near real-time). This enables corrective actions. Extending this principle more broadly, the entire clinical process should be audited continuously. This is exactly the approach taken in “incident learning.” An example of how this works in practice is shown in Figure 1. In this case an incorrectly planned isocenter was identified (panel A vs B), which would have had a significant impact on this patient’s treatment (panel C). As with plan review in clinical trials, the process of incident learning identified this deficiency and corrected it. Experience at Washington University and other groups over the last decade has shown that such incident learning systems (ILS) are feasible even at high volume.2 Given the voluntary nature of reporting it is very encouraging to see that active engagement and high-volume reporting is possible, said Ford.
The movement toward organized incident learning received a boost with the June 2014 release of the Radiation Oncology Incident Learning System™ (RO-ILS), sponsored by ASTRO and AAPM. This system provides a nationwide network that is ready-made for radiation oncology and allows cross-institutional learning. RO-ILS operates under the umbrella of a federally protected patient safety organization (PSO), administered by AHRQ, providing a protective bubble that enables open discussion about how to improve quality.
Six months after the implementation of RO-ILS, some very valuable lessons are already emerging. For example, ensuring that the right patient is treated with the right plan underscores the importance of a checklist and a simple timeout prior to treatment.
“It’s still a work in progress, but we will likely continue to see higher level annual reports that boil down to valuable lessons learned,” said Ford.
Can we prove that engaging in ILS works? Institutions that have adopted ILS have begun to demonstrate its value. The group at the University of Washington, Seattle, demonstrated an improving culture of safety in which people across departments – from technicians to nurses, physicians and medical physicists – operate better in a non-punitive environment, reporting issues and concerns that reveal areas for improvement.3 Another study by investigators at The Ottawa Hospital and Tom Baker Cancer Center examined the benefits of incident learning over a five-year period in which the number of actual non-minor incidents measurably decreased.4 That study also discussed a “reporting index,” a ratio of potential incidents to non-minor actual incidents that appeared to increase over that same time period. That reflected the well-established trend that more reporting incidents and more engagement in incident learning actually reduces the rate of events that harm patients.
“While gold-standard metrics for ILS don’t yet exist, it’s clear that ILS prompts providers to engage and put increased thought behind the safe provision of treatment,” said Ford. “This awareness leads to fewer patient-harm events.”
As powerful as incident learning is, it must be recognized that it does not provide a complete assessment of risk in clinical processes. To address this gap, other quality improvement tools are now coming into wider use, namely process mapping, fault tree analysis, and failure mode and effects analysis (FMEA). The soon-to-be released TG100 Report describes these tools and will roll out a risk-based quality management program based on these tools. FMEA is a core component of this and has been employed effectively by NASA and the U.S. military. FMEA is complementary to ILS as it identifies failure modes – weak points – that typically are not detected through ILS because they are so rare, said Ford. FMEA and TG100 provide a method for judging and quantifying risk and a process for mitigating those risks. There are limitations – FMEA may not identify all possible failure modes. Further, because the incidents are so rare, it is unclear how to quantify the benefits of resulting interventions.
Is FMEA practical to implement in a resource-limited clinic? To date, a number of studies of FMEA have been published in the radiation oncology literature, and several have demonstrated practical, easy-to-use implementations.5-13 A study from the Maastricht Radiation Oncology Clinic examined the effort involved in conducting an FMEA and concluded that it required approximately 19-35 l person-hours in their hands.14
“Short of providing direct proof of improved patient care, FMEA is creating awareness and is being used to refine the design and implementation of quality improvement tools,” said Ford.
In diagnostic imaging, there are a number of initiatives focused on safety as well, using reduced dose as the main patient-safety improvement endpoint. The estimation and reduction of dose has been studied by investigators such as Michael McNitt-Gray and other researchers. Based on such work in the early 2000s, the Image Gently campaign was formed and can be credited with helping to significantly reduce the radiation dose in pediatric imaging scans. The tracking of dose continues to be an important aspect in CT scans and trials. Medicare will soon mandate the recording of CT doses for lung cancer screening, and dose data will be collected via a registry.
What does the future of hold for safety since in medical physics? The science is evolving, notes Ford, but safety research is gaining recognition as an important aspect of medical physics – and an expanded role for medical physicists in healthcare delivery.
“Most studies have focused on the operational aspects of safety and the use of quality assurance tools, while very little research has focused on how effective these tools are and whether they are actually helping patients,” said Ford. “We need to focus on identifying metrics and demonstrating meaningful outcomes. In other words, we need hard data. We’ve got our work cut out for us, but the impact is huge.”
- FitzGerald T, Bishop-Jodoin M, Bosch W, et al. Future vision for the quality assurance of oncology clinical trials. Front Oncol. 2013;Mar 15:3:31.
- Mutic S, Brame R, Oddiraju S, et al. Event (error and near-miss) reporting and learning system for process improvement in radiation oncology. Med Phys. 2010;37(9):5027-5036.
- Kusano A, Nyflot M, Zeng J, et al. Measurable improvement in patient safety culture: a departmental experience with incident learning. Pract Radiat Oncol. 2014;Oct 28 (epub ahead of print).
- Clark B, Brown R, Ploquin J and Dunscombe P. Patient safety improvements in radiation treatment through 5 years of incident learning. Pract Radiat Oncol. 2013;Jul-Sep;3(3):157-63.
- Ford E, Gaudette R, Myers L, et al. Evaluation of safety in a radiation oncology setting using failure mode and effects analysis. Intl J Radiat Oncol Biol Phys. 2009;74:852-858.
- Scorsetti M., Signori C, Lattuada P, et al. Applying failure mode effects and criticality analysis in radiotherapy: Lessons learned and perspectives of enhancement. Radiother Onco. 2010;94:367-374.
- Sawant A, Dieterich S, Svatos M and Keall P. Failure mode and effect analysis-based quality assurance for dynamic MLC tracking systems. Med Phys. 2010;37:6466-6479.
- Perks J, Stanic S, Stern R, et al. Failure mode and effect analysis for delivery of lung stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys.2012;83:1324-1329.
- Ciocca M, Cantone M, Veronese I, et al. Application of failure mode and effects analysis to intraoperative radiation therapy using mobile electron linear accelerators. Int J Radiat Oncol Biol Phys. 2012;82:e305-e311.
- Broggi S, Cantone M, Chiara A, et al. Application of failure mode and effects analysis (FMEA) to pretreatment phases in tomotherapy. J Appl Clin Med Phy. 2013;14:265-277.
- Wilkinson D and Kolar M. Failure modes and effects analysis applied to high-dose-rate brachytherapy treatment planning. Brachytherapy. 2013;12:382-386.
- Cantone M, Ciocca M, Dionisi F, et al. Application of failure mode and effects analysis to treatment planning in scanned proton beam radiotherapy. Radiat Oncol. 2013;8:127-136.
- Ford E, Smith K, Terezakis S, et al. A streamlined failure mode and effects analysis. Med Phys. 2014;Jun 41(6):061709.
- Vlayen A, Evaluation of time-and cost-saving modifications of HFMEA: An experimental approach in radiotherapy. J Patient Safety. 2011;7:165-168.



















